We believe that our mission is to ensure high-quality products and a stable supply.
The manufacturing process for drugs is mainly divided into the “Active Pharmaceutical Ingredients manufacturing process” and the “Formulation manufacturing process.”
The “Active Pharmaceutical Ingredients manufacturing process” is the chemical synthesis of active pharmaceutical ingredients from raw materials through pharmaceutical intermediates.
The “Formulation manufacturing process” is the mixing of pharmaceutical ingredients and pharmaceutical additives (excipients, etc.) to produce the best final form for the drug, such as tablets, capsules, or granules.
At Kotobuki Pharmaceutical Co., Ltd., we use the chemical synthesis technology and unique formulation technology we have developed over many years to domestically produce a large number of “Active Pharmaceutical Ingredients” and “Formulations” at our own factory (located in Nagano).

Both synthetic drug substances and final formulations comply with inspections by overseas regulatory authorities, and we export multiple items. In addition to manufacturing our own products for the domestic market, we also accept contract manufacturing from multiple domestic pharmaceutical companies.
[Production System] ~To foster a quality culture~
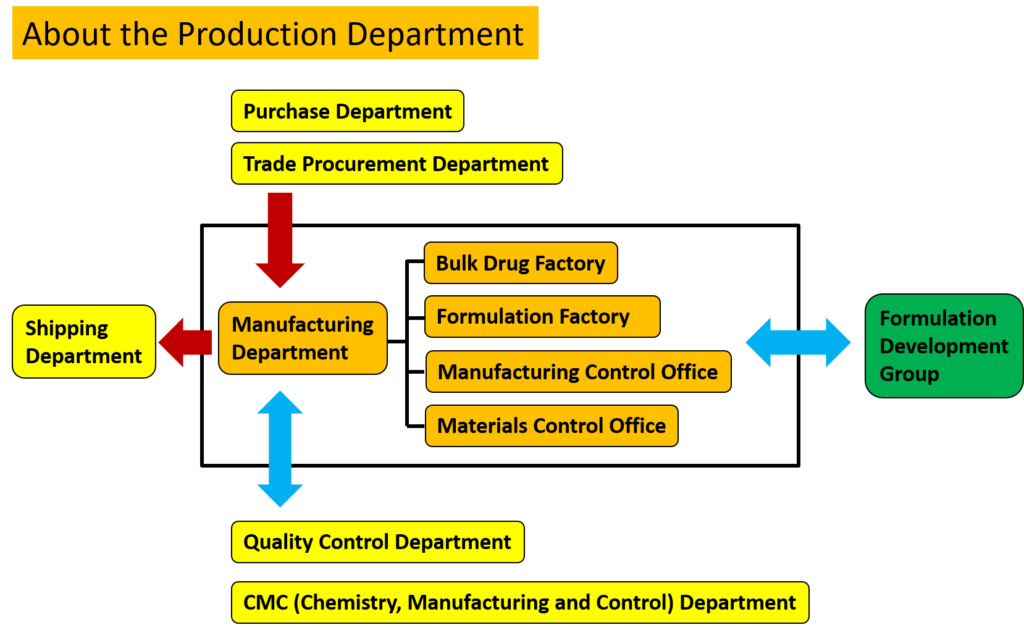
The production system at Kotobuki Pharmaceutical is centered around the “Manufacturing Headquarters,” the “Manufacturing Department,” the “Quality Control Department,” the “CMC (Chemistry, Manufacturing and Control) Department,” and the “Manufacturing Promotion Department,” and is made up of the “Purchase Department,” “Trade Procurement Department,” and “Shipping Department.”
The “Manufacturing Department” has the “Bulk Drug Factory,” “Formulation Factory,” “Manufacturing Control Office,” and “Materials Control Office.” The bulk drug factory manufactures the bulk drug substances used in our pharmaceutical products, and the formulation factory manufactures them (formulates them). The “Manufacturing Control Office” and “Materials Control Office” also handle manufacturing plans and the receipt and disbursement of materials used in manufacturing.
The Manufacturing Promotion Department prepares the manufacturing environment and works to improve work efficiency and quality.
The “Purchase Department” and “Trade Procurement Department” receive the amount required for manufacturing, and the raw materials that pass the quality control office’s quality inspection are handed over to the “Manufacturing Department.”
【About the work of the main departments】
[Active Pharmaceutical Ingredients Factory]
We carry out all manufacturing processes from weighing raw materials ⇒ reaction ⇒ extraction ⇒ concentration ⇒ crystallization ⇒ isolation ⇒ drying ⇒ packaging
in-house.
Specific operations include creating manufacturing-related documents such as procedure manuals and records in accordance with GMP (Good Manufacturing Practice) and carrying out manufacturing operations in accordance with them, thereby ensuring quality and ensuring reproducible manufacturing.
In addition, regarding the manufacturing
environment, we strive daily to improve the safety and workability of our
workers and ensure the stable operation of the bulk drug factory by cleaning
the equipment used, conducting various inspections, and maintaining a hygienic
environment.




~Active Pharmaceutical Ingredients manufacturing facilities~
[Formulation Factory]
We carry out all manufacturing processes from weighing the active pharmaceutical ingredients ⇒ granulation ⇒ tableting ⇒ inspection ⇒ packaging.
As with the active pharmaceutical ingredients factory, we create manufacturing-related documents such as procedure manuals and records in accordance with GMP and carry out manufacturing work in accordance with them, ensuring stable production while guaranteeing quality.
In addition, regarding the manufacturing environment, we strive daily to improve the safety and workability of our workers and ensure the stable operation of our formulation factory by cleaning the equipment used, conducting various inspections, and creating a hygienic environment.





~Formulations manufacturing facilities~
[Manufacturing Promotion Department]
This department is responsible for managing the entire factory, including the introduction of manufacturing equipment, management of the manufacturing line, energy conservation, streamlining and improvement of work, and waste management.
In addition, in cooperation with the quality assurance department, we also consider scaling up from laboratory-scale synthesis in grams to factory-scale production in tons, research the optimization of synthesis processes, synthesis of impurities that may be mixed in during production, and development of standards and test methods.


~Manufacturing Promotion Department facilities~
[Quality Control Office]
The Quality Control Office is a department independent of the manufacturing department.
In accordance with the Pharmaceuticals and Medical Devices Act, GMP, and PIC/S, the Quality Control Office constantly manages and monitors product quality by conducting a wide variety of tests, including raw material acceptance tests, in-process tests, product tests, stability monitoring tests, and trend analysis.
In addition, by feeding back test and analysis results to the manufacturing department, this leads to improvements in quality.
For this reason, the staff in charge always strive to perform precise and accurate work every day.


~Quality Control Office facilities~
[CMC Department]
An abbreviation for Chemistry, Manufacturing and Control, where Chemistry, Manufacturing and Control refer to the management of these. The CMC Department prepares the information related to manufacturing required for drug approval applications. For this reason, we work with the Quality Assurance Department to set control values for the manufacturing process, set specifications for raw materials and reagents, and set the specifications and formulations for active pharmaceutical ingredients, as well as the evaluation methods and grounds for their settings, manage the raw materials for formulations, and evaluate the quality of products.
[Ensuring product reliability]
We have established the QA Department as a direct organization of the Manufacturing Headquarters and are working to improve the reliability of our products. In order to deliver pharmaceuticals that patients and medical professionals can use with confidence, the QA Department has established its own standards and procedures, checked all records related to manufacturing and quality testing, and conducted internal investigations of the manufacturing plant and the quality control department to establish a reliability assurance system for our products.
The QA Department works with the CMC Department to reduce risks related to the quality of pharmaceuticals at each stage of production activities, such as product manufacturing and quality testing, and is continuously working to improve quality. In addition, we work with the Quality Assurance Headquarters to analyze quality information provided by customers, which leads to product quality and the delivery of high-quality pharmaceuticals.
[Human Resources Development and Education System]
The Manufacturing Headquarters not only holds regular in-house training sessions and study sessions, but also utilizes external training systems to develop and educate employees involved in manufacturing and quality control.
For pharmaceuticals, the Ministry of Health, Labor and Welfare has set strict standards for everything from development to manufacturing, shipping, collecting information on side effects, and providing information on proper use. The Manufacturing Headquarters works with the Quality Assurance Headquarters to hold regular monthly study sessions, ensuring that all employees involved in manufacturing and quality control understand and share various guidelines, including the GMP Ministerial Ordinance. In addition, based on the results of analyzing quality information provided by customers, we conduct education and training each time, and work to ensure product quality.